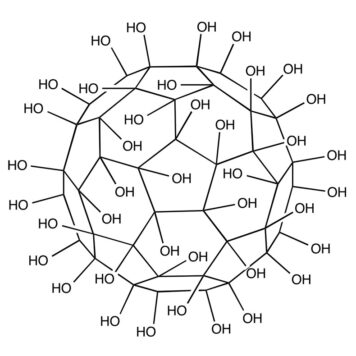
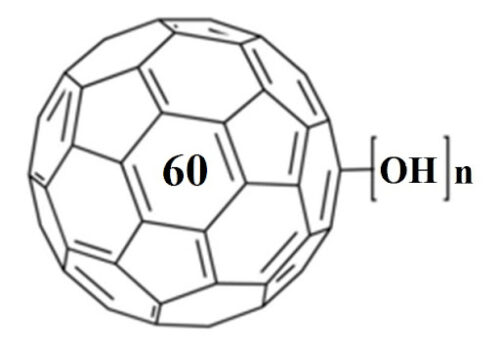
In this work structuring of water and insight into intermolecular interactions between water and fullerenol are studied throughout the process of forming nanoagglomerates at different temperatures applying both experimental and computational approaches. The obtained fullerenol nanoparticles (FNPs) are firstly characterized using dynamic light scattering, atomic force microscopy and transmission electron microscopy. The density, electrical conductivity and dynamic viscosity of aqueous fullerenol solutions are measured in the temperature range of 293.15 to 315.15 K. From the experimental density results other important thermodynamic values, such as apparent molar volumes and the partial molar volumes of water and fullerenol, are also calculated. To support the conclusion derived from the experimental density and calculated volumetric parameters, and to better understand the nature of the interactions with water, molecular dynamics simulations and radial distribution functions are also employed.
Related researches 71 articles










![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)










![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)