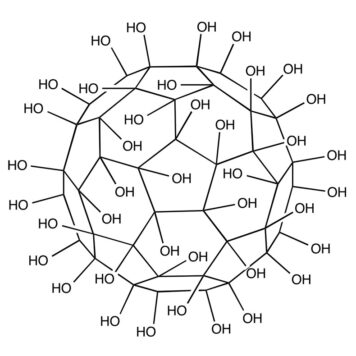
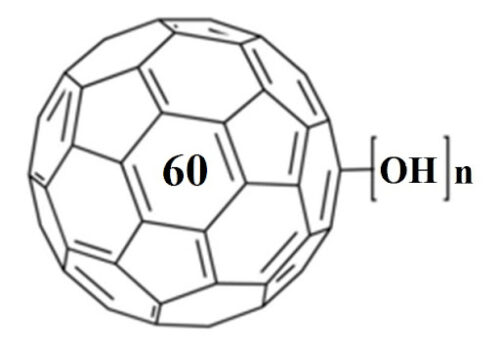
Water-solubilization is the prerequisite to endow the pristinely hydrophobic fullerenes with biocompatibility and biofunctionality, which has been widely applied to derive fullerene-based nanomaterials for biomedical applications. Oxidation reactions using O2 and H2O2 are the most commonly used approaches to this end, through which fullerenols with different structural features can be obtained. Despite the progress in the syntheses and bioapplications of fullerenols, their formation mechanisms and structures at the atomic level, which substantialize their physical properties and biofunctions, have been little understood. Using density functional theory calculations, we comparatively study the mechanisms and product structures for the oxidations of C60, Gd@C60 and Gd@C82 using both O2 and H2O2 as oxidizing agents under both neutral and alkaline aqueous conditions. We predict the formation mechanisms and product structures corresponding to the different synthetic conditions. Briefly, the H2O2 oxidations of C60, Gd@C60 and Gd@C82 under neutral conditions do not occur readily at room temperature because of the high energy barriers, whereas the H2O2 oxidations can readily proceed under alkaline conditions. The oxygen-containing groups of the fullerenols obtained under these conditions include hydroxyl, carbonyl, hemiacetal and deprotonated vic-diol. In contrast, through O2 oxidation under alkaline conditions, the most probable oxygen-containing groups for C60 fullerenols are epoxide and deprotonated vic-diol, and those for Gd@C60 and Gd@C82 fullerenols are hydroxyls and carbonyls. The results explain a wide range of experimental findings reported before. More importantly, they provide atomistic-level insights into the formation mechanisms and structures for various fullerenols, which are of fundamental interest for understanding their biomedical applications in the future.
Related researches 71 articles











![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)










![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)