All living organisms are subject to oxidative stress caused by the formation of free radicals in the body. Most free radicals are aggressive oxidizing agents and their formation within the body can lead to toxic effects. The process of radical formation in the body is constant, but there are factors that increase the amount of radicals in the body, such as ionizing types of radiation and general aging of the body.
Fullerenol has pronounced antiradical properties with low toxicity in the working concentration range.
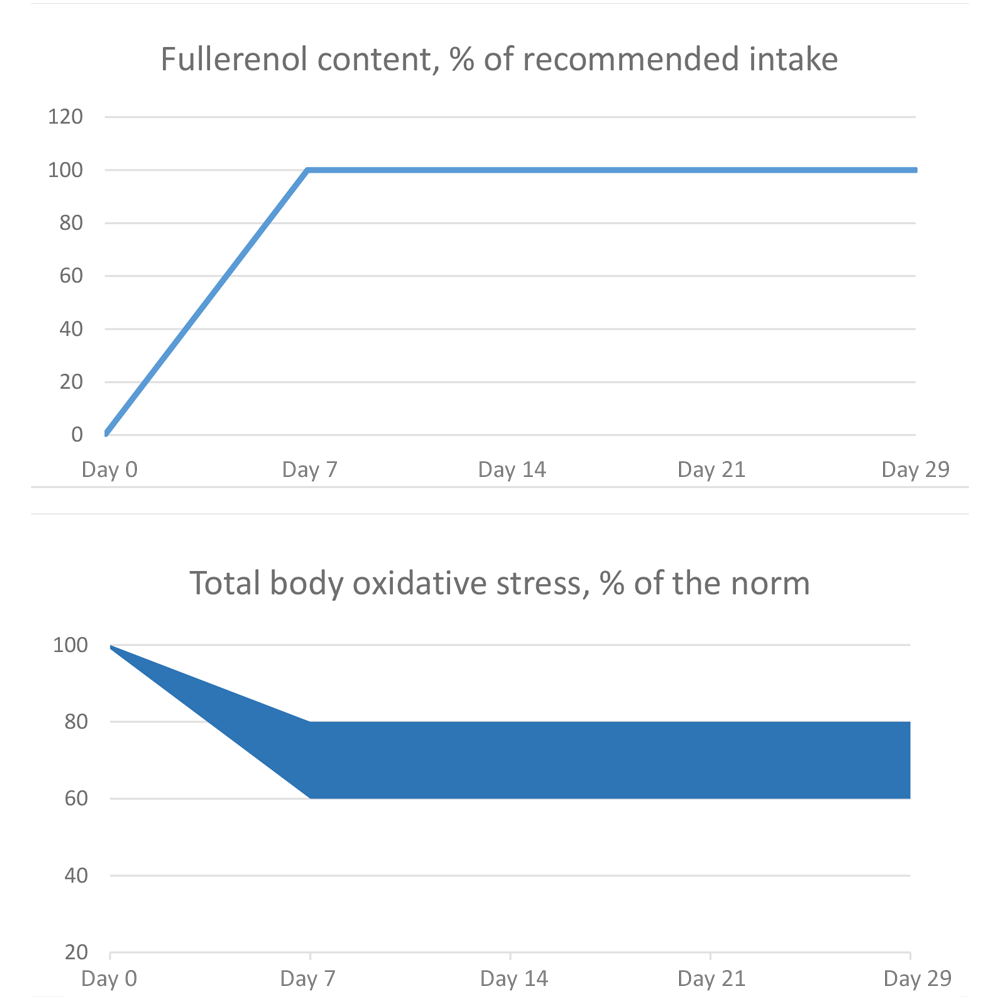
The number of studies that simulate oxidative stress on animals or on laboratory cell lines is large. The conclusions of these studies often correspond to a narrow task set within a particular study. However, the vast majority of them show active reduction of oxidative stress injury factors by an average of 20-40% compared to the control, Fig. 1.
The word “antioxidant” in its strict chemical sense means a substance capable of stopping the chain reaction of free radical formation. To understand the reason for this ability, a free radical should be represented as an abstract molecule with one unpaired (strictly unpaired) electron, and the force with which this unpaired electron tends to get a pair is much higher than the forces of many bonds of the surrounding molecules consisting of paired electrons. A non-oxidant molecule interacting with a free radical gives up one electron from its system of bonds formed by paired electrons, thus becoming a free radical itself. Obviously, the chemical properties of the radical and the initial molecule are strikingly different, which leads to a critical violation of further metabolism of this molecule and the formation of potentially toxic reaction products that are not radicals.
It is generally accepted that free radicals within an individual organism are formed continuously at an approximately constant rate for a variety of reasons. An increase in the number of acts of primary radical molecule formation is often influenced by ionizing irradiation, such as standard radiation background or gamma rays from X-ray machines, ultraviolet radiation from the sun, etc. Hence the community’s interest in antioxidants. An antioxidant molecule is able to interrupt the chain of radical formation reactions if its structure and properties allow it to localize the unpaired electron on itself (a stable radical is formed, which has no high activity) or recombine the unpaired electron, for example, with a water proton.
However, most substances investigated for use as antioxidants are either participants in human metabolism themselves or have toxic effects on the body both by themselves and through the formation of toxic products of their recombination with radicals.
Polyhydroxylated fullerenes (Fullerenols) have a unique set of qualities that coincide well with the basic requirements for antioxidants:
- The structure of the fullerene core allows the formation of relatively stable forms of radicals.
- The large number of hydroxyl groups near the double bonds of the fullerenol molecule allows for recombination of an unpaired electron.
- The specific design of fullerenols does not allow them to “entrench” in metabolic processes, so fullerenols and their “radical recombination” products do not cause toxic and adverse effects in the working concentration range.
The ability of fullerenes to recombine the unpaired electron allows a single fullerenol molecule to remain unchanged by multiple acts of interaction with free radicals. Here, many researchers cite the superiority of the antioxidant activity of fullerenols compared to their analogues by tens or even hundreds of times. It should be understood that certain classes of substances in living organisms have different “propensity” to form free radicals and maintain a chain reaction of their formation, and often the results of quantitative calculations of antioxidant activity are quite difficult to interpret unambiguously.
The topic of studying fullerenols will not exhaust itself any time soon, the supposed mechanisms and methods of use are still proposed for discussion by a huge number of scientists around the world. Nevertheless, at the moment there are already published papers quite clearly confirming the positive effect of fullerenols on organisms under high oxidative stress.
A group of researchers from the Chinese Academy of Sciences cites a study (Zhao et al. 2021) comparing the effects of fullerenol and superoxide dismutase (SOD), an antioxidant enzyme in the mitochondria of all living things. Fullerenol was used as a hydrogel-based ointment; superoxide dismutase was used as a commercially available ointment.
As an oxidative stress, a hard X-ray irradiation was used, with which 4 cm2 of the skin of the laboratory mouse was irradiated. Further, the development of radiodermatitis was observed in five groups of animals (Fig. 2): 1) control group, no irradiation, 2) hard x-ray irradiation (X), 3) hard x-ray irradiation, unmodified hydrogel treatment (X + gel), 4) hard x-ray irradiation, superoxide dismutase ointment (X + SOD), 5) hard x-ray irradiation, 100 mg/kg fullerenol hydrogel (X + Fullerenol + gel). Figure 3 shows the results of histological sections of the affected areas to illustrate the depth of the lesion and skin recovery.
X-ray irradiation in the experiment causes a flurry of free radicals in the skin of mice, which leads to the formation of a pronounced radiodermatitis. The use of fullerenol C60(OH)30 leads to clear positive healing results, and the reason for this is precisely the ability of fullerenols to safely recombine large amounts of free radicals.
Reference:
Zhao, Maoru et al. 2021. “Eco-Friendly and Scalable Synthesis of Fullerenols with High Free Radical Scavenging Ability for Skin Radioprotection.” Small (Weinheim an der Bergstrasse, Germany) 17(37). https://pubmed.ncbi.nlm.nih.gov/34337863/ (December 9, 2022).