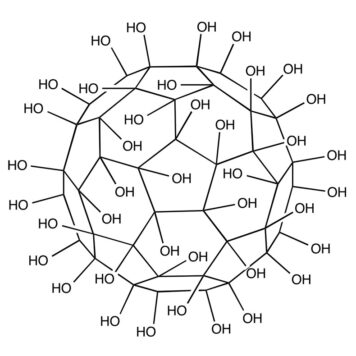
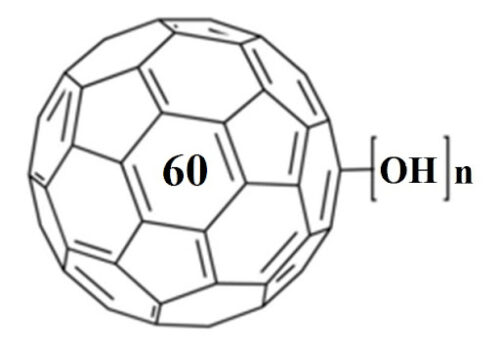
The potential biomedical applications of fullerenol C(60)(OH)(x) (x≈24) have been extensively studied. However, the structural information of the interaction of fullerenol with the bio-system at the molecular level, which is essential for understanding its bioactivity and toxicity, is still missing. In this study, lysozyme was selected as a model protein to investigate the interaction between fullerenol and biomolecules. A strong induced circular dichroism (CD) signal of achiral fullerenol was observed after binding with lysozyme. Activity assay shows that lysozyme activity is inhibited significantly by fullerenol. No heat capacity difference between the folded and unfolded states of lysozyme was measured by differential scanning calorimetry (DSC) in the presence of fullerenol, indicating that fullerenol prefers to bind with the hydrophobic residues. Both experimental and Autodock computational results suggest that the binding site on lysozyme for fullerenol is close to Trp 62, and a π-π stacking interaction might play an important role in binding.
Related researches 71 articles











![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)










![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)