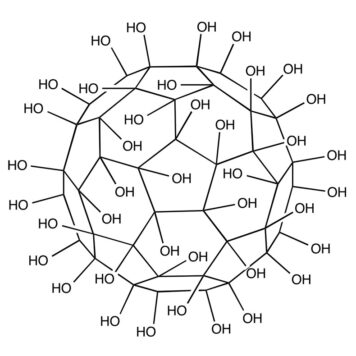
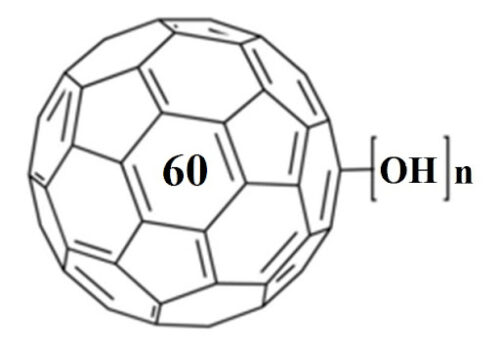
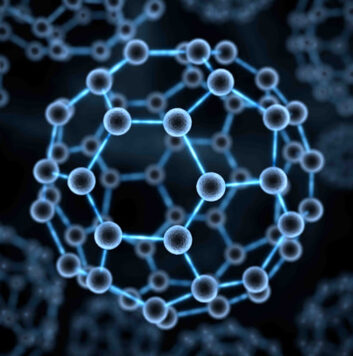
Fullerenes, also known as buckyballs, are spheroidal carbon cages with a molecular formula of C2n#nbsp;(n#nbsp;= 30–41) and a diameter of ~1 nm (1). The high chemical stability of fullerenes can resist any potential metabolic cage-opening process and hence prevents them from degradation in various biological conditions (2). Fullerenes contain hollow interiors that can hold atoms or ions as payloads for various biomedical applications (1). For example, Gd atoms or the trimetallic nitride Gd3N can be trapped inside the cage to form endohedral metallofullerenes denoted as Gd@C2n#nbsp;or Gd3N@C2n, where the “@” symbol refers to the encapsulated nature of the Gd or Gd3N. For the Gd@C2n, the encapsulation of electropositive Gd atom leads to the transfer of electrons from the Gd atom to the electronegative fullerene cage, resulting in a zwitterion [Gd3+@C823-]. For Gd3N@C2n, each Gd atom shares one electron with the N atom and donates the other two electrons to the C80 cage that is in#nbsp;Ih-symmetry to produce a zwitterion [Gd3N6+@C806-] (3, 4). These endohedral metallofullerenes thus lead to the generation of a novel type of contrast agent for magnetic resonance imaging (MRI), which possesses a high biological safety in that the carbon cage completely prevents the release of toxic Gd3+#nbsp;ions into surrounding tissues (5, 6). The surface of fullerenes can be functionalized with a variety of groups or specific ligands; i.e., the addition of hydroxyls or polyethylene glycols (PEG) substantially increases their aqueous solubility (7).
Gd-Fullerenols (Gd@C82(OH)40) are water-soluble Gd endohedral metallofullerenes used with#nbsp;in vivo#nbsp;MRI (8). The encapsulation of Gd has three electrons transferred to the C82 fullerene cage, resulting in zwitterions [Gd3+@C82(OH)403-] in which seven unpaired electrons are located on the 4f-orbital of gadolinium ion and one unpaired electron is located on the fullerene cage. These unpaired electrons contribute to the paramagnetic moment of Gd@C82(OH)40. Nevertheless, the encapsulation of the Gd ion inside the carbon cage prevents direct coordination and exchange of water to Gd3+. The relaxation enhancement of Gd@C82(OH)40#nbsp;is therefore quite different from the classic inner-sphere mechanism in conventional Gd chelates such as Gd-DTPA but depends on its paramagnetic moment and intermolecular aggregation effect (1). The aggregation leads to an increase in the rotational correlation time and alterations in the water exchange kinetics, which both influence the resultant relaxivity.
Related researches 71 articles











![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)










![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)