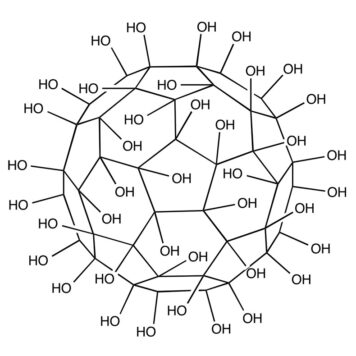
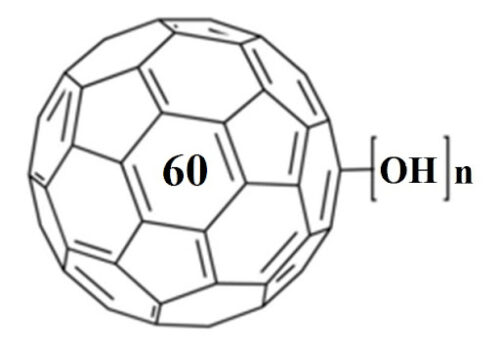
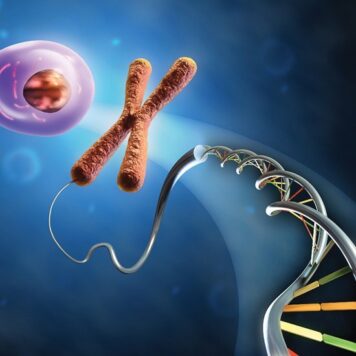
Nanoscale assembly is an area of research that has vast implications for molecular design, sensing, nanofabrication, supramolecular chemistry, catalysis, and environmental remediation. Here we show that poly(amidoamine) (PAMAM) dendrimers of both generations 1 (G1) and 4 (G4) can host 1 fullerenol per 2 dendrimer primary amines as evidenced by isothermal titration calorimetry, dynamic light scattering and spectrofluorometry. Thermodynamically, the interactions were similarly spontaneous between both generations of dendrimers and fullerenols, however, G4 formed stronger complexes with fullerenols resulting from their higher surface charge density and more internal voids, as demonstrated by spectrofluorometry. In addition to hydrogen bonding that existed between the dendrimer primary amines and the fullerenol oxygens, hydrophobic and electrostatic interactions also contributed to complex formation and dynamics. Such hybrid of soft and condensed nanoassembly may have implications for environmental remediation of discharged nanomaterials and entail new applications in drug delivery.
Related researches 71 articles











![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)









![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)