Various positive properties
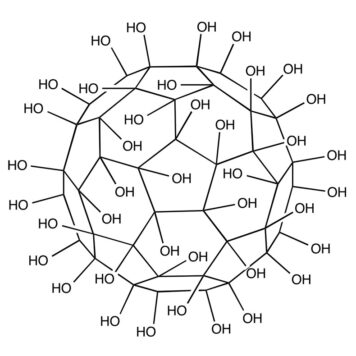
We investigate the impact of hydroxyl groups on the properties of C(60)(OH)(n) systems, with n = 1, 2, 3, 4, 8, 10, 16, 18, 24, 32 and 36 by means of first-principles density functional theory calculations. A detailed analysis from the local density of states has shown that adsorbed OH groups can induce dangling bonds in specific carbon atoms around the adsorption site. This increases the tendency to form polyhydroxylated fullerenes (fullerenols). The structural stability is analyzed in terms of the calculated formation enthalpy of each species. Also, a careful examination of the electron density of states for different fullerenols shows the possibility of synthesizing single molecules with tunable optical properties.
Related researches 71 articles

Cancer / Transport / Delivery
Various positive properties
Exploring the World of Fullerenols: A Deep Dive into Their Potential Medical Use
Various positive properties
Fullerenol has pronounced antiradical properties in the working concentration range
Various positive properties
The transcriptome profile of RPE cells by the fullerenol against hydrogen peroxide stress
Various positive properties
Toxicity and Antioxidant Activity of Fullerenol C60,70 with Low Number of Oxygen Substituents

Various positive properties
Exploiting the physicochemical properties of dendritic polymers for environmental and biological applications

Various positive properties
Impacts of fullerene derivatives on regulating the structure and assembly of collagen molecules
Various positive properties
INHIBITORY POTENTIAL OF POLYHYDROXYLATED FULLERENES AGAINST PROTEIN TYROSINE PHOSPHATASE 1B
Various positive properties
Effect of fullerenol surface chemistry on nanoparticle binding-induced protein misfolding
Various positive properties
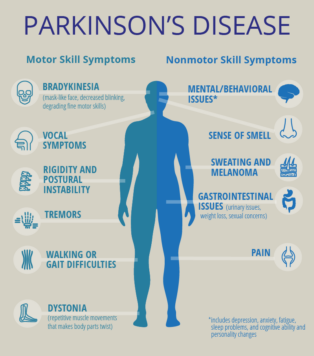
The neuroprotective effect of fullerenols on a model of Parkinson’s disease in Drosophila melanogaster

Various positive properties
Effect of fullerenol nanoparticles on oxidative stress induced by paraquat in honey bees
Various positive properties
Facile synthesis of highly water-soluble fullerenes more than half-covered by hydroxyl groups
Various positive properties
Interaction of fullerenol with lysozyme investigated by experimental and computational approaches

Various positive properties
Facile synthesis of isomerically pure fullerenols and formation of spherical aggregates from C60(OH)8
Various positive properties
Influences of the size and hydroxyl number of fullerenes/fullerenols on their interactions with proteins
Various positive properties
The properties of small fullerenol cluster (C60(OH)24)7: computer simulation

Various positive properties
The structural studies of fullerenol C60(OH)24 and nitric oxide mixture in water solvent – MD simulation
Various positive properties
Production of monoclonal antibodies against fullerene C60 and development of a fullerene enzyme immunoassay
Various positive properties
Mechanism of taq DNA polymerase inhibition by fullerene derivatives: insight from computer simulations

Various positive properties
Polyhydroxylated C60 fullerene (fullerenol) attenuates neutrophilic lung inflammation in mice

Various positive properties
Morphologically virus-like fullerenol nanoparticles act as the dual-functional nanoadjuvant for HIV-1 vaccine
Various positive properties
Fullerenol C₆₀(OH)₃₆ could associate to band 3 protein of human erythrocyte membranes
Various positive properties
Synthesis and Characterization of Hydroxyapatite/Fullerenol Nanocomposites

Various positive properties
Investigation of work of adhesion of biological cell (human hepatocellular carcinoma) by AFM nanoindentation

Various positive properties
Self-assembling, reactivity and molecular dynamics of fullerenol nanoparticles
Various positive properties
Fullerenol C60(OH)24 increases ion permeability of lipid membranes in a pH-dependent manner

Various positive properties
Novel green PVA-fullerenol mixed matrix supported membranes for separating water-THF mixtures by pervaporation
![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)
Various positive properties
Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy
Various positive properties
Increasing the Resistance of Living Cells against Oxidative Stress by Nonnatural Surfactants as Membrane Guards
Various positive properties
Fullerenol C 60(OH) 36 protects human erythrocyte membrane against high-energy electrons

Various positive properties
Molecular Semiconductor Surfactants with Fullerenol Heads and Colored Tails for Carbon Dioxide Photoconversion
Various positive properties
Fullerenol Nanoparticles Eradicate Helicobacter pylori via pH-Responsive Peroxidase Activity









![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)