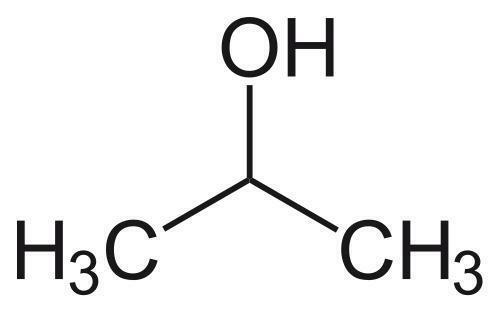
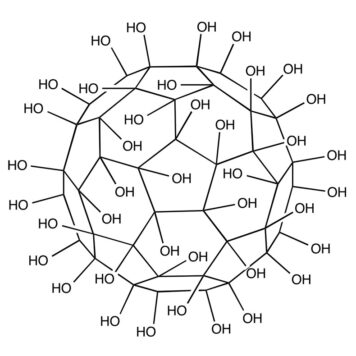
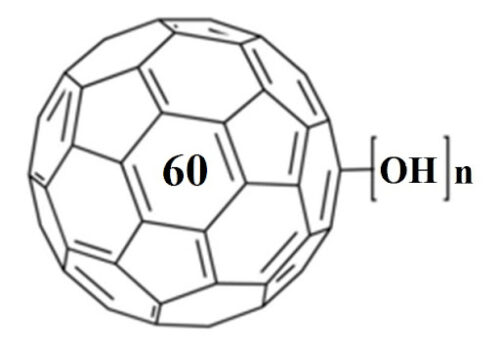
A fullerenol-based synthetic sweetness receptor model, consisting of polyhydroxy groups for potential hydrogen bond donor along with a spherical hydrophobic center, was proposed according to the widely accepted sweetness hypothesis. An isothermal titration calorimetry (ITC) technique was used to study mimetic interaction of this sweet receptor model with a series of sweeteners having increasing sweetness intensity. The results showed that ITC is an effective method to provide thorough and precise characterization of the energies of molecular complex formation. Binding of all of the studied sweeteners with fullerenols was found through two sets of site models. More heat was released from sweeter synthetic compounds binding with fullerenols than from less sweet carbohydrates. The results imply that hydrogen bond formation is necessary for the sweeteners to bind to the fullerenol receptor in the first stage, whereas hydrophobic effect and conformation changes that lead to favorable entropy changes occur in most cases. The preliminary results of this study help to cover the lack of information about the thermodynamic basis of understanding of the initiation of the sweet sensation. It also adds complementary physicochemical measurements available for comparison with the sweetness hypothesis. On the other hand, a correlation between the thermodynamic parameters and sweetness intensity has been made as well, which exhibits potential as a useful tool in sensory analysis.
Related researches 71 articles











![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)










![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)