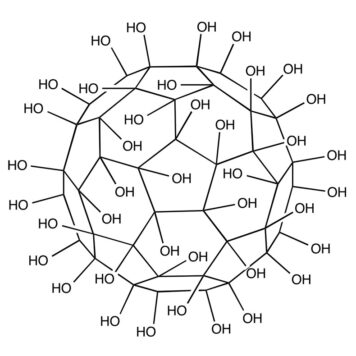
In spite of extensive studies on their preparation and application, the precise structure of fullerenols remains unknown. Fullerenols used in all the known studies are complicated mixtures of compounds. It is impossible to isolate isomerically pure fullerenols even after exhaustive purification procedures. Thus, the exact number of OH groups and their relative locations on the cage are not determined. Hemiketal moieties are present in some cases.[6] Fullerenols prepared by reaction of C60 with aqueous NaOH solution were shown to be structurally and electronically complex radical anions.[7] To understand details of their bioactivity and develop practical fullerene-based medicines, the purity and identity of fullerenols is of crucial importance. New methods are needed to prepare isomerically pure multihydroxylated fullerenes. Here we report the preparation of the first isomerically pure multihydroxylated fullerene by reactions involving fullerene mixed peroxides.
Related researches 71 articles










![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)










![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)