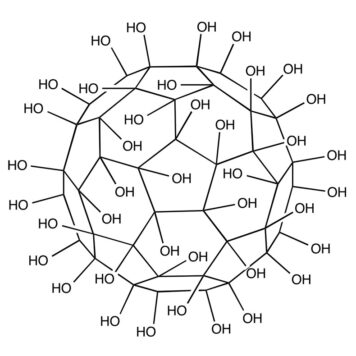
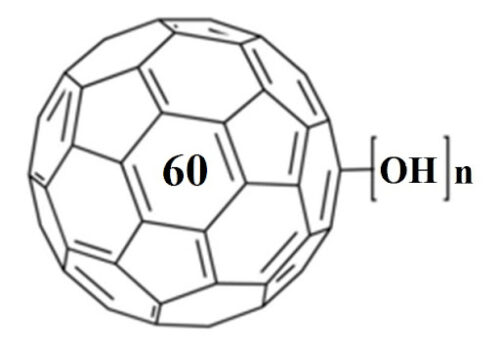
Reactive oxygen species (ROS) are one of the most important intermediates in chemical, photochemical, and biological processes. To understand the environmental exposure and toxicity of fullerenes better, the production and consumption of ROS (singlet oxygen, superoxide, hydrogen peroxide, and hydroxyl radicals) by Buckminster fullerene (C(60) ) and fullerenol were investigated in aqueous systems. Fullerenol exhibits higher photoproduction efficiency of singlet oxygen and superoxide than aqueous suspensions of C(60) aggregates (aqu/nC(60) ), and this higher efficiency results in higher steady-state concentrations of these two ROS. Transmission electron microscopy indicates that the C(60) molecules in aqu/nC(60) are much more closely packed than the C(60) cages in fullerenol. These observations provide additional evidence that the lower ROS production efficiency of aqu/nC(60) is attributable primarily to efficient self-quenching of C(60) triplet states. Production of singlet oxygen by aqu/nC(60) is accelerated by increasing oxygen concentration and in part is sensitized by fluorescent photoproducts that accumulate during irradiation. The fullerenes react slowly with singlet oxygen (second-order rate constant <4 × 10(5) M(-1) s(-1) ), but react rapidly with hydroxyl radicals (second-order rate constants of 5.4 × 10(9) and 4 × 10(8) M(-1) s(-1) for aqu/nC(60) and fullerenol, respectively). These results show that environmental conditions, including light exposure and oxygen concentration, have the potential to impact the generation of toxic ROS by fullerenes.
Related researches 71 articles











![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)










![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)