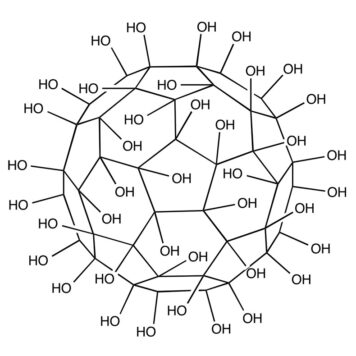
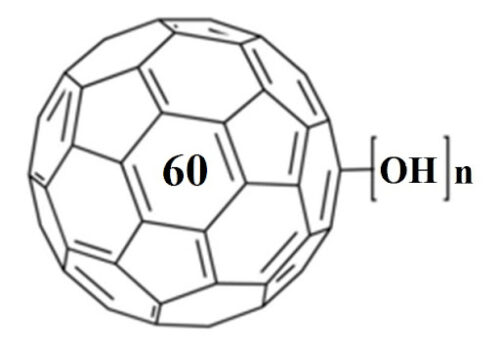
Traditional approaches for nucleic acids detection require prior amplification of target genes, while nanomaterials-aided DNA biosensors are very magnificent but still suffer from the nanomaterial acquirement and limited sensitivity (above picomolar level). Herein, fullerenol C60(OH)25, a representative fullerene derivative, was employed as a photoelectrochemical (PEC) nanoprobe to achieve discrimination and ultrasensitive detection of amplification-free single-stranded DNA (ssDNA) down to sub-femtomolar level. The bonded hydroxyl groups with intense density endowed fullerenol to directly recognize and capture ssDNA-AuNPs via the hydrogen bonding interactions (H-bonds), leading to a sharply decreased photocurrent with quenching efficiency up to 85%, which could be attributed to the photo-generated electrons on the conduction band of fullerenol (-4.66 eV) preferentially migrating to the Fermi level of AuNPs (-5.1 eV) rather than the electrode. In the presence of target gene (mutant human p53 gene fragment), the H-bonds between fullerenol and ssDNA were competitively depleted during the base pairing process of complete hybridization between ssDNA and target, making double-stranded DNA-AuNPs (dsDNA-AuNPs) depart so that the photocurrent powerfully recovered. On basis of the photocurrent variation before and after target introduction, this proposed simple, rapid and ultrasensitive PEC biosensor for amplification-free target gene detection illustrated a wide liner ranged from 1 fM to 100 pM and a detection limit of 0.338 fM. This work presented an ingenious strategy for the discrimination and ultrasensitive detection of nucleic acids, and the well-designed PEC biosensor was further conducive to the impetus of clinic diagnostics.
Related researches 71 articles











![Inhalable gadofullerenol/[70] fullerenol as high-efficiency ROS scavengers for pulmonary fibrosis therapy](https://biofullerene.com/wp-content/uploads/2022/12/istockphoto-12925559-440x356.jpg)










![Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated sp 2 C-H Bond Activation and Hydroxylation](https://biofullerene.com/wp-content/uploads/2022/12/2978543-356x356.png)