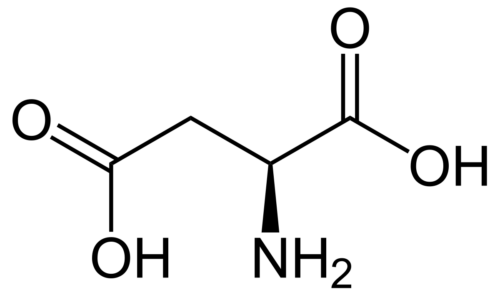
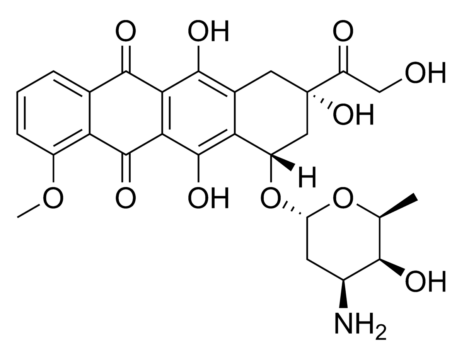
Polyhydroxylated fullerenols especially gadolinium endohedral metallofullerenols (Gd@C82(OH)22) are shown as a promising agent for antitumor chemotherapeutics and good immunoregulatory effects with low toxicity. However, their underlying mechanism remains largely unclear. We found for the first time the persistent uptake and subcellular distribution of metallofullerenols in macrophages by taking advantages of synchrotron-based scanning transmission X-ray microscopy (STXM) with high spatial resolution of 30 nm. Gd@C82(OH)22 can significantly activate primary mouse macrophages to produce pro-inflammatory cytokines like IL-1β. Small interfering RNA (siRNA) knockdown shows that NLRP3 inflammasomes, but not NLRC4, participate in fullerenol-induced IL-1β production. Potassium efflux, activation of P2X7 receptor and intracellular reactive oxygen speciesare also important factors required for fullerenols-induced IL-1β release. Stronger NF-κB signal triggered by Gd@C82(OH)22 is in agreement with higher pro-IL-1β expression than C60(OH)22. Interestingly, TLR4/MyD88 pathway but not TLR2 mediates IL-1β secretion in Gd@C82(OH)22 exposure confirmed by macrophages from MyD88(-/-)/TLR4(-/-)/TLR2(-/-) knockout mice, which is different from C60(OH)22. Our work demonstrated that fullerenols can greatly activate macrophage and promote IL-1β production via both TLRs/MyD88/NF-κB pathway and NLRP3 inflammasome activation, while Gd@C82(OH)22 had stronger ability C60(OH)22 due to the different electron affinity on the surface of carbon cage induced by the encaged gadolinium ion.
Related researches 41 articles




![Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy](https://biofullerene.com/wp-content/uploads/2022/11/istockphoto-65584859-356x356.jpg)