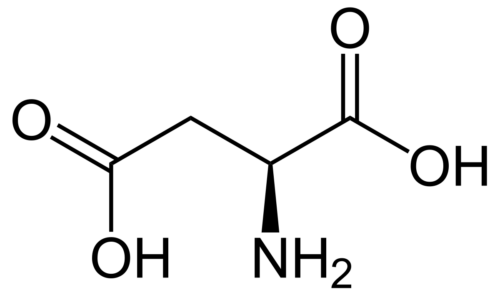
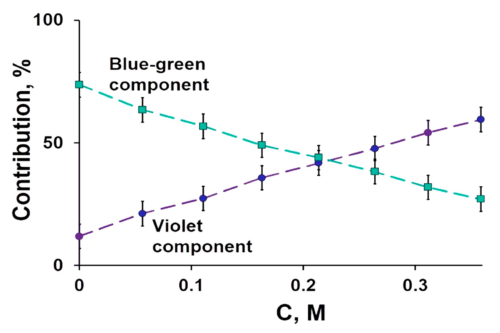
Herein, A549 tumor cell proliferation was confirmed to be positively dependent on the concentration of Fe3+#nbsp;or transferrin (Tf). Gd@C82#nbsp;(OH)22#nbsp;or C60#nbsp;(OH)22#nbsp;effectively inhibited the iron uptake and the subsequent proliferation of A549 cells. The conformational changes of Tf mixed with FeCl3#nbsp;, GdCl3#nbsp;, C60#nbsp;(OH)22#nbsp;or Gd@C82#nbsp;(OH)22#nbsp;were obtained by SAXS. The results demonstrate that Tf homodimers can be decomposed into monomers in the presence of FeCl3#nbsp;, GdCl3#nbsp;or C60#nbsp;(OH)22#nbsp;, but associated into tetramers in the presence of Gd@C82#nbsp;(OH)22#nbsp;. The larger change of SAXS shapes between Tf+C60#nbsp;(OH)22#nbsp;and Tf+FeCl3#nbsp;implies that C60#nbsp;(OH)22#nbsp;is bound to Tf, blocking the iron-binding site. The larger deviation of the SAXS shape from a possible crystal structure of Tf tetramer implies that Gd@C82#nbsp;(OH)22#nbsp;is bound to the Tf tetramer, thus disturbing iron transport. This study well explains the inhibition mechanism of Gd@C82#nbsp;(OH)22#nbsp;and C60#nbsp;(OH)22#nbsp;on the iron uptake and the proliferation of A549 tumor cells and highlights the specific interactions of a nanomedicine with the target biomolecules in cancer therapy.
Related researches 41 articles




![Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy](https://biofullerene.com/wp-content/uploads/2022/11/istockphoto-65584859-356x356.jpg)