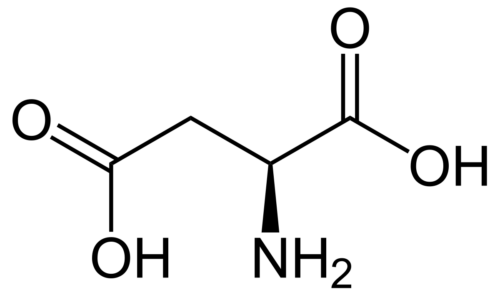
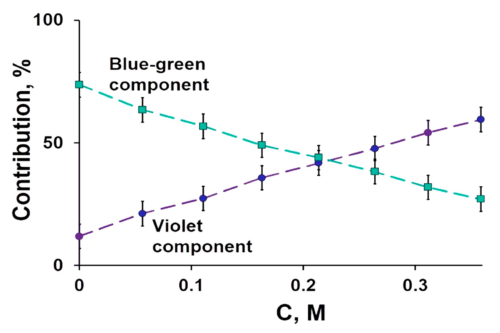
The interaction between Gd@C82(OH)22#nbsp;and serum albumin (HSA and BSA) were investigated by spectroscopic analysis. From the characteristic feature of fluorescence quenching spectra at different temperatures, the inherent binding information including quenching mechanism, association constants, number of binding site, fraction of initial fluorescence and basic thermodynamic parameters were calculated. The binding of Gd@C82(OH)22#nbsp;to serum albumin caused strong quenching of protein intrinsic fluorescence and the structural changes of serum albumin. At lower concentrations, Gd@C82(OH)22#nbsp;was likely to rise fluorescence quenching of serum albumin through individual static quenching process by forming a ground-state complex, while dynamic and static coexisting quenching mechanism occurred in high concentration. Bimolecular quenching (Kq) value is twice the diffusion-controlled quenching constant (2.0 × 1010#nbsp;L mol-1#nbsp;s-1); binding sites of BSA were slightly more than those of HAS, and all of them reached to 1; the distance r between donor and acceptor was found to be 3.1494 nm and 3.6479 nm for HSA and BSA, respectively, both of which were fewer than 7 nm. It is confirmed that binding interaction for proteins in the presence of drugs was strong, the binding ratio was 1:1, and non-radiative energy transfer from protein to drug was extremely high probability in lower density. Binding process of Gd@C82(OH)22-HSA was driven mainly through van der Waals forces and hydrogen bonding formation, however more likely to be electrostatic interaction involved in the Gd@C82(OH)22-BSA binding process; Binding sites of Gd@C82(OH)22#nbsp;to serum albumin were near tryprophan (HSA) and tyrosine residues (BSA), respectively. Moreover, a theoretical model of predicting the binding rate of drug to serum albumin was estimated, further analyzed that the binding rate was dynamically altered in various dose of protein and drug. Overall, these results provide potentially significant information for elucidating the distribution, transportation, the apparent relationship between pharmacologic activity and total plasma drug concentration as well as anti-carcinogenic activity and mechanisms in vivo.
Related researches 41 articles




![Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy](https://biofullerene.com/wp-content/uploads/2022/11/istockphoto-65584859-356x356.jpg)