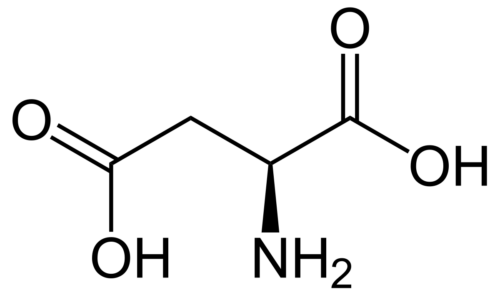
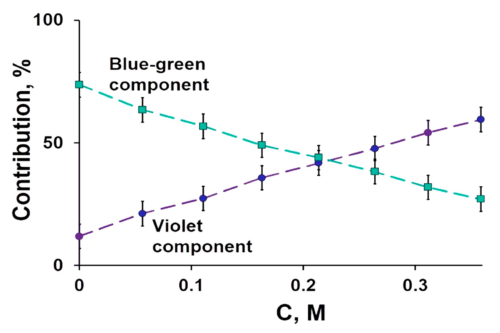
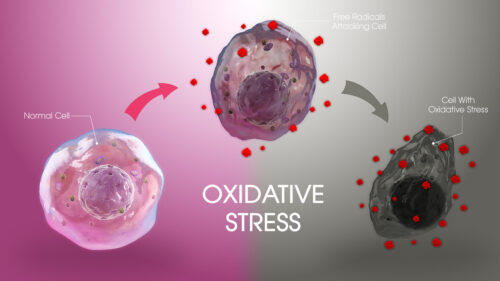
Fullerene-protein interaction studies have been a key topic of investigation in recent times, but the lower water solubility of fullerene somewhat limits its application in the biological system. In this work, we have compared the activities of fullerene and its water-soluble hydrated form, that is fullerenol, on ribonuclease A (RNase A) under physiological conditions (pH 7.4). The interaction studies of fullerene and fullerenol with protein suggest that the binding depends on the hydrophobic interactions between the protein and the ligand. In addition, fullerene and fullerenol slow down the ribonucleolytic activity of RNase A through noncompetitive and mixed types of inhibition, respectively. This precisely gives the idea about the ligand-binding sites in RNase A, which has further been explored using docking studies. Both these nanoparticles show a reduction in dityrosine formation in RNase A caused due to oxidative stress and also prevent RNase A dimer formation to different extents depending on their concentration.
Related researches 41 articles




![Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy](https://biofullerene.com/wp-content/uploads/2022/11/istockphoto-65584859-356x356.jpg)