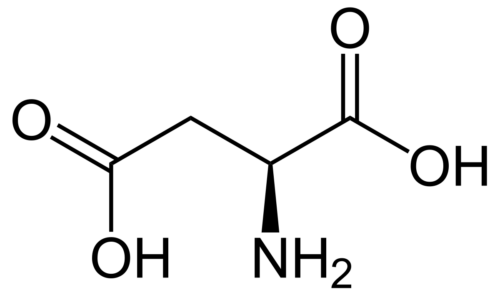
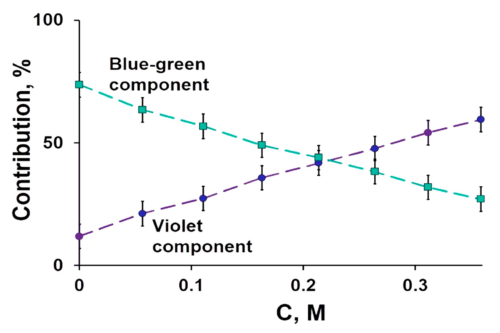
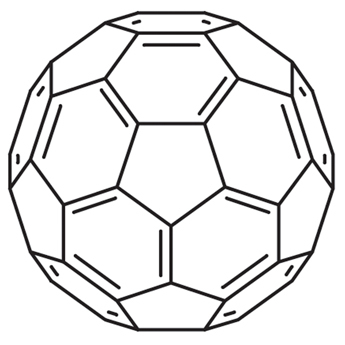
Chemotherapy as a conventional cancer treatment suffers from critical systemic side effects, which is generally considered as the consequence of reactive oxygen species (ROS). Fullerenes have been widely studied for their excellent performance in radicals scavenging. In the present study, we report a solid-liquid reaction to synthesize fullerenols and their application as ROS scavengers in chemotherapy protection. The solid-liquid reaction is carried out without catalyst and suitable for mass production. The novel [60]/[70] fullerenols show a high stability in water, and the [70] fullerenols (C70-OH) exhibit radical scavenging capability superior to that of [60] fullerenols (C60-OH) in chemotherapy protection. The mouse model for single and reduplicative chemotherapy-induced liver injury demonstrates their protective effects in the chemotherapeutic process, which is confirmed by histopathological examinations and hematological index. The increase of the hepatic l-glutathione (GSH) level and downregulated expression of the cytochrome P-450 2E1 (CYP2E1) give the possible mechanism associated with the impact of fullerenols on the metabolism of doxorubicin. The novel fullerenols may be promising protective agents to satisfy the demand for future clinical chemotherapy.
Related researches 41 articles