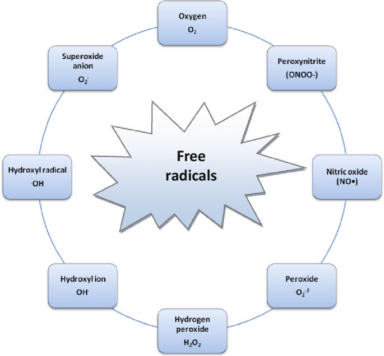
Fullerenols are nanosized water-soluble polyhydroxylated derivatives of fullerenes, a specific allotropic form of carbon, bioactive compounds, and perspective basis for drug development. Our paper analyzes the antioxidant activity and toxicity of a series of fullerenols with different number of oxygen substituents. Two groups of fullerenols were under investigation: (1) C60Oy(OH)x, C60,70Oy(OH)x, where x + y = 24-28 and (2) C60,70Oy(OH)x, Fe0,5C60Oy(OH)x, Gd@C82Oy(OH)x, where x + y = 40-42. Bioluminescent cellular and enzymatic assays (luminous marine bacteria and their enzymatic reactions, respectively) were applied to monitor toxicity in the model fullerenol solutions and bioluminescence was applied as a signaling physiological parameter. The inhibiting concentrations of the fullerenols were determined, revealing the fullerenols’ toxic effects. Antioxidant fullerenol’ ability was studied in solutions of model oxidizer, 1,4-benzoquinone, and detoxification coefficients of general and oxidative types (DGT#nbsp;and#nbsp;DOxT) were calculated. All fullerenols produced toxic effect at high concentrations (>0.01 g L-1), while their antioxidant activity was demonstrated at low and ultralow concentrations (<0.001 g L-1). Quantitative toxic and antioxidant characteristics of the fullerenols (effective concentrations, concentration ranges,#nbsp;DGT, and#nbsp;DOxT) were found to depend on the number of oxygen substituents. Lower toxicity and higher antioxidant activity were determined in solutions of fullerenols with fewer oxygen substituents (x + y = 24-28). The differences in fullerenol properties were attributed to their catalytic activity due to reversible electron acceptance, radical trapping, and balance of reactive oxygen species in aqueous solutions. The results provide pharmaceutical sciences with a basis for selection of carbon nanoparticles with appropriate toxic and antioxidant characteristics. Based on the results, we recommend, to reduce the toxicity of prospective endohedral gadolinium-fullerenol preparations Gd@C82Oy(OH)x, decreasing the number of oxygen groups to x + y = 24-28. The potential of bioluminescence methods to compare toxic and antioxidant characteristics of carbon nanostructures were demonstrated.
Related researches 23 articles