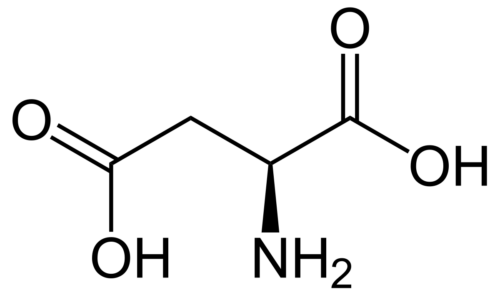
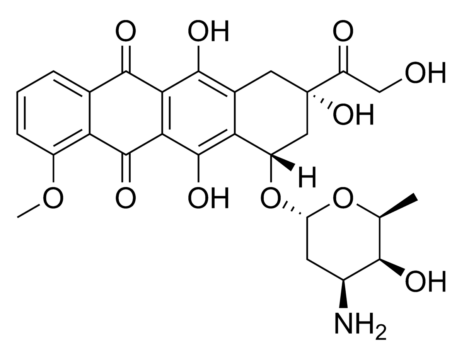
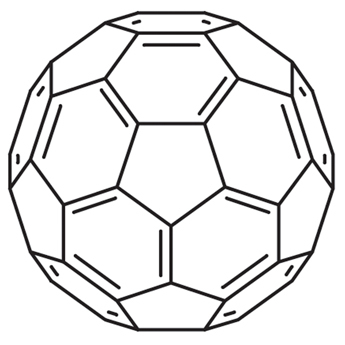
In the present study, we report the novel application of polyhydroxylated fullerenes (fullerenols) in cancer drug delivery. The facile synthetic procedure for generating multiple hydroxyl groups on the fullerene cage offers scope for high drug loading in addition to conferring hydrophilicity. Doxorubicin, a first line cancer chemotherapeutic, was conjugated to fullerenols through a carbamate linker, achieving ultrahigh loading efficiency. The drug-fullerenol conjugate was found to be relatively stable in phosphate buffer saline but temporally released the active drug when incubated with tumor cell lysate. The fullerenol-doxorubicin conjugate suppressed the proliferation of cancer cell-lines in vitro through a G2-M cell cycle block, resulting in apoptosis. Furthermore, in an in vivo murine tumor model, fullerenol-doxorubicin exhibited comparable antitumor efficacy as free drug without the systemic toxicity of free doxorubicin. Additionally, we demonstrate that the fullerenol platform can be extended to other chemotherapeutic agents, such as the slightly water-soluble cisplatin, and can emerge as a new paradigm in the management of cancer.
Related researches 41 articles




![Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy](https://biofullerene.com/wp-content/uploads/2022/11/istockphoto-65584859-356x356.jpg)