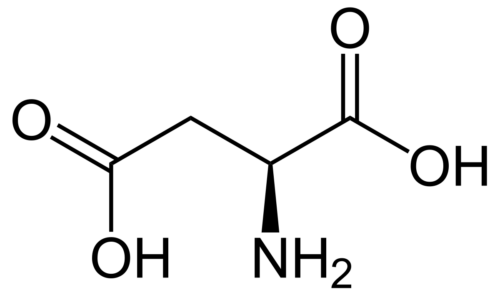
Adoptive immunotherapy is a highly effective approach for cancer treatment. Several potential adoptive immunotherapies have high (though reversible) toxicities with disappointing results. Polyhydroxylated fullerenols have been demonstrated as promising antitumor drugs with low toxicities. In this study, we investigate whether polyhydroxylated fullerenols (C60(OH)22 and Gd@C82(OH)22) contribute to cancer immunotherapy by regulating macrophages. Our results show that fullerenols treatment enhances mitochondrial metabolism, phagocytosis and cytokine secretion. Moreover, activated macrophages inhibit the growth of several cancer cell types. It is likely that this inhibition is dependent on an NF-κB-mediated release of multiple cytokines. Using a lung metastasis model, we also show that autologous macrophages greatly suppress cancer cell metastasis to lung when they are activated by C60(OH)22 and Gd@C82(OH)22. More importantly, Gd@C82(OH)22 are shown to have stronger ability than C60(OH)22 to improve the macrophage function, which shed light on the rational design for nanomedicine and clinical application.
Related researches 41 articles




![Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy](https://biofullerene.com/wp-content/uploads/2022/11/istockphoto-65584859-356x356.jpg)